|
In the first step (Preparation and characterisation of mono- and bis-pyrazoles from the corresponding chalcones) of the bilateral research project SAMPHENO was prepared mono- and bis-pyrazoles by a condensation-ciclisation reaction of some mono- and bis-chalcones which contain phenothiazine units with methylhidrazine. The chalcones with phenothiazine units which are used as precursors was obtained by methodes which are described in literature1,6,7 . In this way was synthesised the compound 10-methyl-3-[1- methyl -3-(phenyl)-1H-pyrazol-5-yl]-10H-phenothiazine 2a (scheme 1).
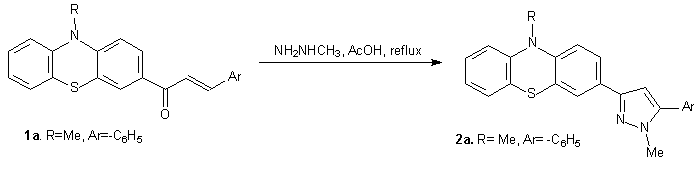 Scheme 1 Scheme 1
The synthesis of the pyrazol 10-(n-buthyl)-3-[1-methyl-3-(ferrocenyl)-1H-pyrazol-5-yl]-10H-phenothiazine 2b, which was studied previously by the research group which participate to the project, was restart according to following reaction (scheme 2):
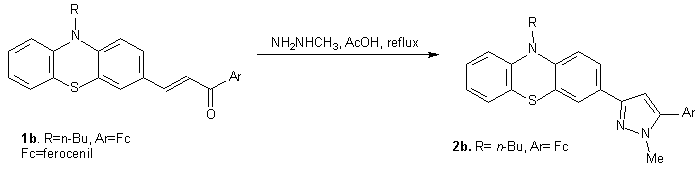 Scheme 2 Scheme 2
The aromatisation by oxidation of the pyrazolinic nucleus is stricken by the substituent from position one. In accordance with a study1 was estabished that the nitrogen acilat atom is an deficitar electron which decrease the oxidability of the nucleus. The effect +Iof the methyl group from position 1 increase the density of the electrons to the nitrogen and implicitly the oxidability of the nucleus. A characteristic probleme of the pyrazoles and pyrazolines synthesis from chalcones is the possibility of the formation a two position isomers2,3,4,5.
In the case of pyrazol 2b the determination of the position pyrazolic methyl group was realize with help the NMR of high resolution by NOE experiment .
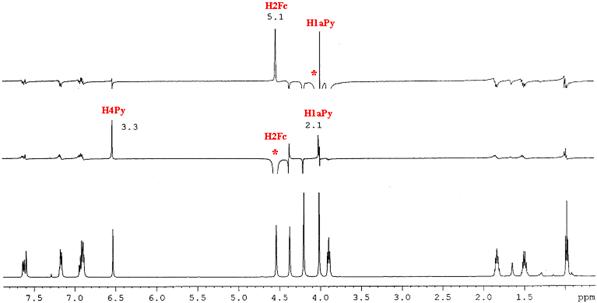
Spectrum 1H-NMR NOE of the compound 2b., at 500MHz in CDCl3
Through the iradiation with resonance frequence of a singlet from δ= 4,00 ppm (H1aPy) was observed an increase in intensity of the semnal from δ= 4,53 ppm (H2Fc) respectively through the iradiation with resonance frequence of a forenamed singlet was observed the increase in intensity both a pyrazolinic proton semnal δ= 6,52 ppm (H4Py) and proton from methyl group (H1aPy). These observations prove the proximity of the methyl group from position one of the pyrazole from ferrocenyl group.
Was realize the synthese of some symmetrical dipyrazoles of type: 10-alchil-3,7-bis(1-methyl-5-aril-1H-pyrazol-3-yl)-10H-phenothiazines 4, 5, 6 (scheme 3) with potential applications as bidentate ligands, in which the pyrazolic groups have effect orto-director and in this way these make possible the metallation of the position 4 and 6 of the phenothiazine.
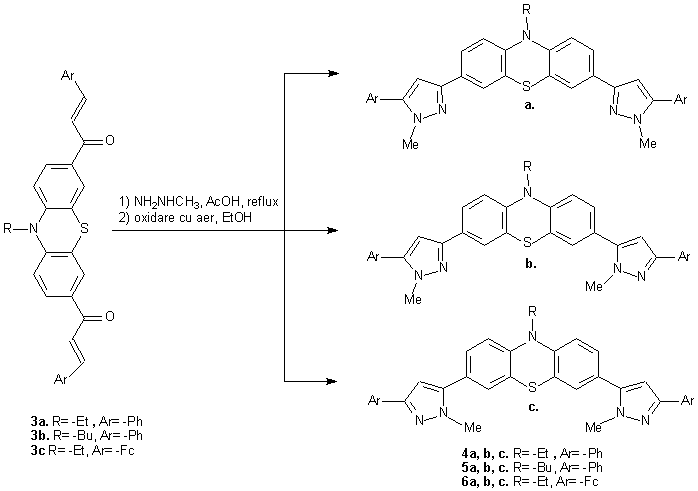 Scheme 3 Scheme 3
In the metallation reaction (such as. litiation), the pyrazol units which are grafted in the position 3,7 of the phenothiazine nucleus, manifest a orto-director effect and in this way, by totaling of this effect with effect of the sulphur atom from heterocyclic strusture, make possibile regioselective metallation of the position 4 and 6 of the phenothiazinic substrate.
In the case of the bis-pyrazoles of type 4 was isolated only majority product 4a, and the separation of the compound 4b and 4c was not possible due the chromatographic behavior and the similar solubility of those two derivatives in the used solvents. The aromatization of the pyrazolic nucleus was realized by oxidation with air (in ethanolic solution) of the pyrazolinic derivatives mixture which was separated from the methylhidrazine excess and from the solvent (acetic acid) in which was realized the ciclisation.
Was observed that in all the studied pyrazole synthesis was happenned the total conversion of the chalcones which was used as starting material to adding an enough quantity of methylhidrazine.
The reactions progress was followed by TLC (using as eluent dicloromethane/acetone 10:1 or 5:1 v/v). The reaction time was in general of 8h which was enough for the products aromatization. The products isolation was realized by column chromatography (Kieselgel tipe 9385, using as eluent dicloromethane/acetone, 10:1) and recristalisation from EtOH-n-hexane (about 2:1 v/v). The compound 4a was separated from the pyrazoles mixture (4a+4b+4c) by recurrent recristalisations from mixture of MeOH-n-hexane (about 1:1 v/v) in which is less soluble than 4b and 4c.
The regioisomers isolation of type 5a and 6a, which are useful for future studies, and their structural analysis is in progress.
Also was synthesised, pyrazolinic derivatives starting to bis-phenothiazinyl-chalcone 1,5-bis-(10-methyl-3-phenothiazin-3-yl)-1,4-pentadien-3-one 7 by condensation reaction with phenil hidrazine and respectively methylhidrazine in acetic acid , at reflux; was obtained: N-methyl-3-[(E)vinyl-2-(10-methyl-3-phenothiazin-3-yl]-5-(10-methyl-3-phenothiazin-3-yl)]-1H-pyrazole 8, respectivelyN-phenil-3-[(E)vinyl-2-(10-methyl-3-phenothiazin-3-yl]-5-(10-methyl-3- phenothiazin-3-yl)]-1H-pyrazole 9 (scheme 4)
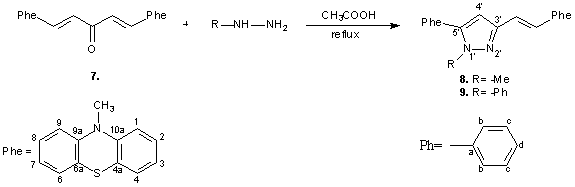
Scheme 4
Behind ciclisation reaction with phenilhidrazine was obtained alongside pyrazole, the accordingly pyrazoline, but in a very small ratio.
The structural caracterisation of the synthesised compound was realized by spectroscopic methodes. It was registered and attributed the FT-IR, UV-Vis and NMR spectras of high resolution (1D and 2D H-NMR, 13C-NMR) which follow to be presented in the experimental part.
|
 Scheme 1
Scheme 1 Scheme 2
Scheme 2
 Scheme 3
Scheme 3