|
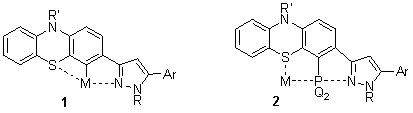
We plan the metallation of the phenothiazinyl-substituted pyrazoles with organolithium-, organopalladium- and organoplatinum reagents to obtain novel pincer type metallacycles 1. The lithio derivatives can be converted into phosphines which are expected to behave as bidentate S- and P-donor ligands. The donor character in complexes 1 and 2 can be tuned via the R- and Ar-substituents (including the strongly electron-releasing ferrocenyl group) of the interacting pyrazole ring. Other possibilities provided by the lithio intermediates include the introduction of electrophiles into position 4 which are potential precursors of a series of novel phenothiazines in a variety of cross-coupling- and condensation reactions. The same reaction sequence can be utilized for the preparation of novel multifunctionalised analogues and bimetallic complexes from the corresponding bis-pyrazoles.
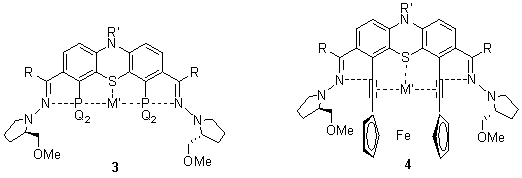
The 3,7-diacylphenothiazines can also be condensed with chiral N-amino-2-methoxymethylpyrrolidines affording bis-hydrazones of C2 symmetry. Their multistep transformations involving directed metallation at positions 4 and 6 may afford a variety of chiral bimetallic ligands and complexes. The 4,6-bis-lithiated intermediates are also supposed to react with different electrophiles to obtain novel 3,4,6,7-tetrasubstituted phenothiazines some of them being multidentate ligands as the diphosphine ligands in complexes 3. The 4,6-dihalo derivatives are planned to be cross-coupled with different reagents including 1,1’-bis-ethinylferrocene resulting in chiral ferrocenophane ligands with co-operating S-donor phenothiazine unit. These ligands seem to be suitable to binding a variety of low-valence transition metals to form a novel class of chiral catalysts 4.
If necessary, the reactions planned to be used in the synthetic work will be accelerated by microwave irradiation of which effect on the substrate- and regioselectivity will also be studied.
The target compounds will be subjected to detailed structural analysis by combined spectroscopic methods (IR, NMR), X-ray diffraction and quantum-chemical calculations with special regard to conformation and metal-ligand- and metal-metal interactions.
|

