|
OBJECTIVES Ob.1 Production, isolation and purification of various wt- and mutant PAL and PAM. ∑Wt-PAL from various mesophiles
and termophiles will be produced by fermentation.
The enzyme activity and stability will be tested and optimal condition for
highest reaction rate will be established. Cloning known and novel bacterial
useful PALís into the most proper hosts for larger scale productions. Ob2. Immobilization of PAL and
PAM, and whole cells hosting PAL/PAM production. We plan to develop robust
immobilization techniques with isolated enzymes as well as with whole cells
producing MIO-containing enzymes. The immobilization method can
basically determine the reusability and activity of enzymes,
moreover it can affect the substrate specificity of the biocatalysts. Several
immobilization methods including adsorption binding onto surfaces, entrapment
or cross-linking will be performed and compared their feasibility and
efficiency. Ob3. Development of biocatalytic procedures mediated by PAL/PAM The broad substrate tolerance of PAL
will be exploited further by using wild-type or mutant native or immobilized mesophile and extermophile
enzymes for the efficient production of both enantiomers of various aryl-alanines. Natural or engineered cells will be also
checked as potential biocatalysts. Batch and continuous flow processes will
be compared as efficiency. A continuous flow sequential procedure will be
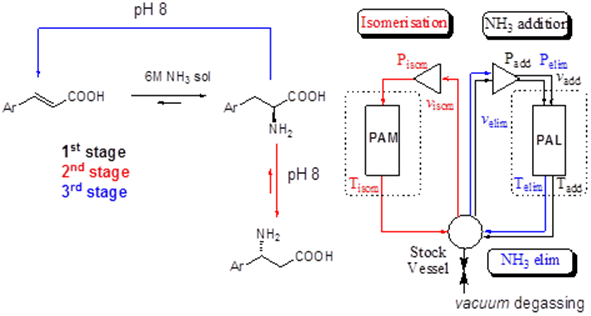
developed for the simple and efficient production of unnatural
β-phenylalanine analogs using as starting material various aryl-cinnamates as shown. In the first stage PAL catalysed ammonia addition (6M NH3 water
solution) will provide L-arylalanines. After the
reaction will ceased the solution will be degassed and the pH will be
adjusted to 8. In the second stage the PAM catalysed
izomerization will provide the corresponding
β-arylalanine. Finally the unreacted L-arylalanie will be transformed into the starting arylacrylate.† |