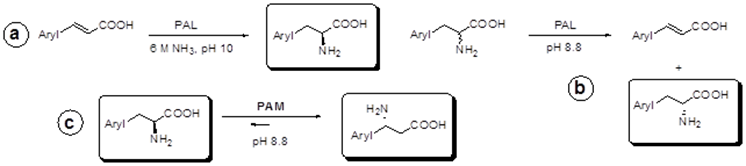ABSTRACT
The goal of the proposed project is
to explore/develop heat stable/protease resistant native and immobilized wild
type (from various sources) and mutant phenylalanine ammonia- lyase (PAL) and –mutase (PAM) for
biocatalysis. Based on the considerations regarding
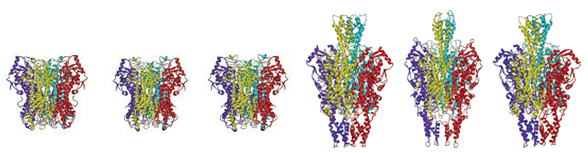
the stability differences between eukaryotic and prokaryotic PAL, a modified
PAL construct, containing the catalytic domain of the eukaryotic PAL and the
C-terminal domain of a bacterial PAL, will be assembled. The strategy of
removing the destabilizing C-terminal region will be used to obtain more heat
and protease resistant PAM enzymes, which are known so far only from eukaryotic
sources. Successful execution of the proposed work will provide highly active,
stable enzymes with broad substrate tolerance for the production of unnatural enantiopure (R)- and (S)-alfa- and beta-arylalanines,
which are interesting building blocks for chemical and pharmaceutical
synthesis. The use of immobilized PAL and PAM will permit the design of easily
scaled-up continuous flow procedures for industrial manufacturing of the target
compounds.