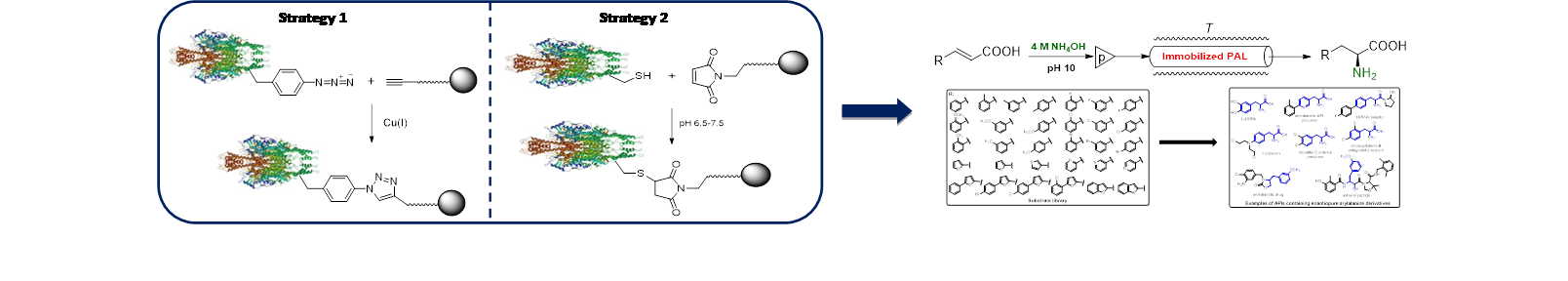
Abstract
Phenylalanine ammonia lyases (PALs) catalyse
the non-oxidative deamination of l-phenylalanine
to trans-cinnamic acid, while in the
presence of high ammonia concentration the reverse reaction occurs. The
PAL-mediated large scale synthesis of non-natural amino acids is limited,
mainly due to the decreased operational stability. The main goal of the SIR-PAL
project is to develop highly stable and active phenylalanine ammonia
lyase-based biocatalysts through site-specific covalent immobilization
techniques employing: 1) site-specific
incorporation of unnatural amino acids and 2) maleimide/thiol
coupling of engineered enzymes. These immobilization methods afford
highly stable biocatalysts (through strong covalent binding to the support) and
allow modulation of enzymatic activity (through control of orientation of the
enzyme attached to the support, by proper selection of binding site). The novel
biocatalysts will be applied in enzymatic kinetic resolutions and asymmetric
additions in batch and continuous-flow packed-bed
reactors, with the aim of developing efficient biocatalytic procedures for
obtaining optically pure industrially relevant unnatural amino acids.