Abstract
The synthesis of natural and unnatural aromatic amino acids in homochiral form is highlighted by the significant interest towards these building blocks in the development of therapeutic peptides and proteins. An attractive enzymatic route to enantiomerically pure α- or β- aromatic amino acids involves the use of aromatic ammonia lyases (PALs) and aminomutases (PAMs).
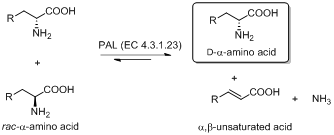
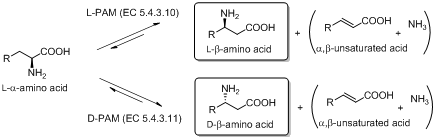
The currently known aromatic ALs and AMs use an autocatalically formed 5-methylene-3,5-dihydro-imidazole-4-one (MIO) prosthetic group, and show high structural and sequence similarities. The state of art suggests an unexplored synthetic potential of MIO-enzymes and highlights the need to define and expand their substrate scope.
The structure of the MIO-group:
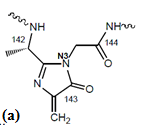
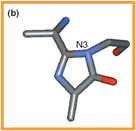
The catalytic site of HAL enzyme containing the MIO group:
In this frame the present project proposes to create a MIO-enzyme toolkit, consisting from:
- a MIO-enzyme library
- a novel sensitive fluorescence-based high throughput activity screening method
- a significantly sized non-natural substrate library
The MIO-toolkit will:
- expand and define the substrate tolerance of the MIO-enzymes towards a large, pallet of non-natural substrates, broadening the biocatalytic applications of these enzymes;
- permit to researchers to further expand the enzyme and susbtrate library developed, the activity assay allowing the fast and rapid screening of the enzyme activities towards the desired substrate;
- give access to the directed evolution of these enzymes, through the developed high-throughput activity assay.