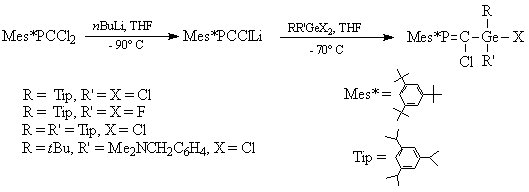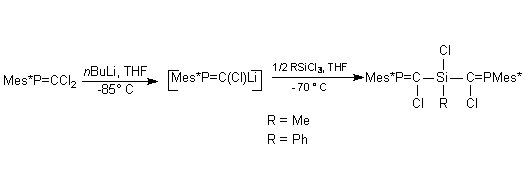Heteroallenic derivatives
The chemistry of phosphaalkenes and phosphaallenes has received increasing interest from the scientific community in the last decades. However, heteroallenic systems containing both heavier group 14 and 15 elements are not well known, due to their instability, but they are interesting systems, from the point of view of fundamental research, as well as for their potential applications. Thus, the study of doubly bonded derivatives of the kind mentioned before provides insight in the nature of multiple bonding for heavier main group elements, a challenge that issued from the great difference between the behavior of carbon and that of its congeners. As far as the potential applications are concerned, compounds containing the E=C=P unit can be excellent precursors in the synthesis of organic polymers, that would have special properties due to the presence of the heteroatoms. An improvement of the optical, mechanical and especially the electrical properties of this kind of polymers is foreseen.
1. Phosphagerma-, phosphasila- and phosphastanapropenes –precursors in the synthesis of heteroallenic systems
The general synthetic route leading to unsaturated compounds containing the E14=C=P involves the synthesis of substituted phosphaalkenes, according to the scheme below. Subsequent action of tBuLi and elimination of lithium halides would afford the desired allenes.
Thus, several germyl-substituted phosphaalkenes were obtained starting from the sterricaly hindered Mes*P=CCl2, and employing dihaloderivatives of germanium (scheme 1). Bulky organic groups on the germanium atom were also used in order to afford a better sterric protection of the Ge=C double bond in the phosphagermaallene.
Scheme 1
The preparation of a phosphasilaallene of a new type which could be stabilized both by steric and electronic effects was attempted, considering a conjugation between the allenic moiety and a C=P double bond, leading to a derivative of the type –P=C=Si(R)-R'C=P–. The corresponding precursors were synthesized according to scheme 2:
Scheme 2
The synthesis of the first arsenyl-substituted bis(methylene)phosphorane, was carried out starting from an aryl-[bis(trimethylsilyl)-methylene]-phosphorane (scheme 3).
Scheme 3
Only one 31P-NMR signal is found for the reaction product, so only the E isomer is formed with the chlorine atom in a trans position with respect to the supermesityl group, indicating an attack of nBuLi on the less sterricaly hindered side of the P=C double bond.
In order to increase the synthetic utility of phosphasilaallene, we tried to attach at the silicon atom a chlorine atom which could allow functionalisation of the Si=C=P system. The other group at Si was a 9-methylfluorenyl group intended to provide extrasteric protection for the Si=C double bond. A novel trichlorophosphasilapropene was prepared by coupling a phosphacarbenoid with dichloro(9-methylfluorenyl)silane (scheme 4).
Scheme 4
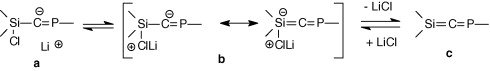
Surprisingly, whereas the elimination of lithium halide occurred at low temperature in the case other heteroallenic precursorssubstituted at Si and Ge by aryl groups, no elimination was observed in the case of this trichlorophosphasilapropene. Calculations on the [Cl3-nHnSi-C = PH]− anion at the RHF/6-31 + G** level show that the increasing number of chlorine atoms at the silicon increases the negative charge (natural charges) on the central carbon from −1.224 (n = 3) to −1.393 (n = 0) . This is in favour of form a whereas form b should be necessary to form the double bond (scheme 5), according to a study of Wiberg on compounds of the type Si(X)-C(Li).
Scheme 5
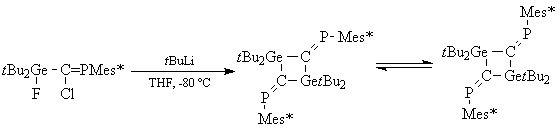
To have a better understanding about the non elimination of LiCl, molecular orbital calculations (RHF/3-21G* level) have been carried out to determine the relative stability of the conformers of the simplified system Cp′Cl2Si-C(Li) = PMes* (Cp′ = 1-methylcyclopentadienyl). The most stable isomer shows a strong interaction between the lithium atom and the pi systems of Mes* and Cp′ with short distances between Li and the centroid of these aromatic systems. This lithium-π interaction also favours form a. However, the chlorosilyllithium derivative can be considered as a synthetic equivalent of the phosphasilaallene RClSi=C=PMes*.
2. Theoretical studies
Theoretical investigations of the heteroallenic systems are of great importance, because they can afford information on their intimate electronic structures and also provide ways to stabilize the two double bonds. In our group,DFT theoretical studies on the stability of germa-, sila- and arsaphosphaallenes were carried out, in an attempt to explain the small number of such derivatives described to date.
In the case of phosphagermaallenes, it was shown that increasing hindrance around the Ge=C=P unit by using very bulky groups (like Mes, Tip, tBu or Mes*) allows the isolation of phosphagermaallenes. Thus, bulkier substituents will better protect the multiple bonds in the opened structures while increasing the strain of the 3-atom rings, favoring the 1,3-phosphagermaallenes. As another way to stabilise the P=C=Ge unit consists in the use electronic effects, an NBOstudy was carried out in order to identify the influence that the nature (electropositive or electronegative) of several substituents would have on the strength of the C=Ge bond. Model compounds HP=C=GeR2 and HP=C=GeRR' were investigated (R = BH2, CH3, SiH3, NH2, OMe, F; R' = H). It was found that the main interaction contributing to the weakening of the C=Ge bond is a transfer of electron density from the lone pair of the phosphorus atom to the molecular antibonding orbital localized on the Ge-C bond (scheme 6).
Scheme 6
Substituents with atoms bearing lone pairs or vacant p orbitals induce other types of charge transfer which also result in the weakening of the Ge=C bond order, while groups containing group 14 elements like Si and C are found to be "inert". A distinction between aromatic and aliphatic substituents could not be made at this level of the theory, but influence of the sterric effect was already proven experimentally. So, bulky organic and silyl substituents are the best choice for the stabilization of the Ge=C=P unit.
Similar studies on silaphosphagermaallenes, 1,3-diarsaallenes and arsaphosphaallenes provide usefull information on what the best choice of substituents on the heteroatoms is, in order to stabilize the heteroallenic unit.
3. Digermacyclobutanes with exocyclic C=P and C=P=S Double Bonds
Experimental work on germaphosphaallenes led us to the synthesis of new 1,3-digermacyclobutanes substituted by two arylphosphanylidenic groups (scheme 7). Two geometrical isomers, cis and trans, with respect to the -P=C•••C=P- axis were identified.
Scheme 7
Quantum-chemical calculations were carried out on model compounds of the digermacyclobutanes, at the B3LYP/6-31G(d) level and show a slight preference for the trans isomer. However the effect of the solvent reverses this trend. Further theoretical investigations revealed that although there seems to be no effective conjugation between the two P=C bonds throughout the cycle; the high stability of these derivatives can be explained through stabilizing interactions throughout the 4-atom ring and the doubly-bonded phosphorus atom.
The solid-state structure of both isomers was characterized by X-ray diffraction. The sulfurated derivatives (cis and trans forms), the first bis(methylenethioxophospho-ranes), were easily obtained by the reaction of the digermacyclobutanes with S8 at 60 °C. The solid-state structures of these derivatives were also determined.
Relevant publications:
New Halo Compounds of Si, P, As and Sb bearing a Bulky Substituted Fluorenyl Group, L. Baiget, M. Bouslikhane, J. Escudié, G. Cretiu Nemes, I. Silaghi-Dumitrescu, L. Silaghi-Dumitrescu,Phosphorus, Sulfur, Silicon and Related Elements, 2003, 178(9), 1949.
Phosphasila- (germa- and arsa-)allenes -P=C=E (E = Si, Ge, As) and arsa- and diarsaallenes -As=C=E' (E' = C, As) (review), J. Escudie, H. Ranaivonjatovo, M. Bouslikhane, Y. El Harouch, L. Baiget, G. Cretiu Nemes, Russian Chemical Bulletin, Int. Ed. 2004, 53, 1020. (review).
The surprisingly stable lithiochloro compound RCl2Si-C(Li)=PMes* : a synthetic equivalent of a chlorophosphasilaallene, G. Cretiu Nemes, H. Ranaivonjatovo, J. Eescudie, I. Silaghi-Dumitrescu, L. Silaghi-Dumitrescu, H. Gornitzka, Eur. J. Inorg. Chem., 2005, 1109.
New halo compounds of silicon and tin, potential precursors of >E=C=P- heteroaallenic systems, P. M. Petrar, G. Nemes, I. Silaghi-Dumitrescu, L. Silaghi-Dumitrescu, Studia Univ. Babes-Bolyai, Chemia, 2006, LI (1), 77.
Synthesis of new bromo-stannanes: toward unsaturated tin derivatives, P. M. Petrar, G. Nemes, I. Silaghi-Dumitrescu, L. Silaghi-Dumitrescu, Studia Univ. Babes-Bolyai, Chemia, 2006, LI (2), 33.
New Digermylalkenes and Digermylalkynes: [1,3]-Chlorine Shift in Organogermanium Chemistry?, G. Nemes, J. Escudie, I. Silaghi-Dumitrescu, H. Ranaivonjatovo, L. Silaghi-Dumitrescu, H. Gornitzka, Organometallics, 2007, 5136.
1,3-Digermacyclobutanes with exocyclic CLP and CLPLS double Bonds, P. M. Petrar, G. Nemes, I. Silaghi-Dumitrescu, H. Ranaivonjatovo, H. Gornitzka, J. Escudie, Chem. Commun., 2007,40, 4149.
Synthesis and Characterization of the First Arsanylbis(methylene)phosphorane (Me3Si)2C=P(Mes*)=C(Cl)-As(F)Mes*, P. M. Petrar, G. Nemes, L. Silaghi-Dumitrescu, I. Silaghi-Dumitrescu, J. Escudié, H. Gornitzka, H. Ranaivonjatovo, Rev. Roum. Chim.,2007 , (1), 3.
Secondary interactions in heteroallenic system with P=C=E units, G. Nemes, I. Silaghi-Dumitrescu, P. M. Petrar, R. Septelean, L. Silaghi-Dumitrescu, Studia Univ. Babes-Bolyai, Chemia, 2007, LII (1), 3.