Abstract
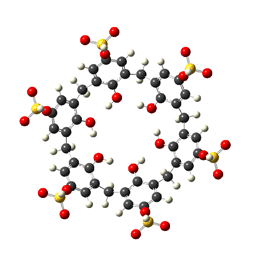
The complexation ability of calix[4]arene tetrasulfonate towards some alkaline cations (Li+, Na+, Cs+) as well as for the neutral molecule (GTP-14564) which exhibits a promising biological activity against leukemia as a tyrosine kinase inhibitor was studied. For complexation of larger alkaline earth cations (Ca2+, Ba2+) calix[6]arene hexasulfonate was used as a host with enlarged cavity. Thermodynamic parameters of complex formation were determined by ab initio calculations at the RHF/6-31G(d) level applying Basis Set Superposition Error (BSSE) corrections. Conformational analysis shows that the Na+ cation becomes encapsulated in the cavity of the calixarene whereas the Li+ and Cs+ cations coordinates to the lower or upper rims of calixarene, respectively. Bonding and energetic properties were analyzed by Natural Bond Orbital (NBO) methodology. NBO calculations reveals that dipolar interactions are more important in the case of complexes with ions than with the neutral GTP molecule in which dispersion forces seem to be pronounced in the stabilization. Trends in the thermochemistry derived from vibrational analysis of alkaline complexes of calixarenes exhibit differences according to the fitting ratio of guests inside the cavity. We could also conclude that calix[4]arene tetrasulfonate is a suitable candidate to be a delivery agent for GTP.