Radu Silaghi-Dumitrescu
NITRIC OXIDE REDUCTION BY P450NOR
P450NOR
is a heme enzyme employed by fungi to reduce nitric
oxide, a toxic intermediate which is produced in these organisms upon nitrate
reduction. The electrons required for this reduction are provided by NADH:
![]()
Known steps of the P450nor reaction, from
experiment:
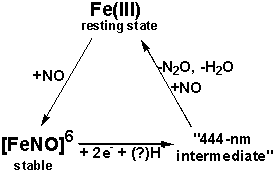
We
have engaged in computational investigations of the P450NOR, employing density
functional calculations (DFT) and molecular mechanics-based docking. Emphasis
has been placed on establishing the structure of the experimentally-observed
‘444-nm intermediate’; candidates for this species have included, among others,
such structures as [FeNO]7, [FeNO]8, [FeNOH]8,
[FeN(H)O]8, or [FeN(H)OH]8.
The issue of outer-sphere vs. inner-sphere electron transfer has also been
examined.
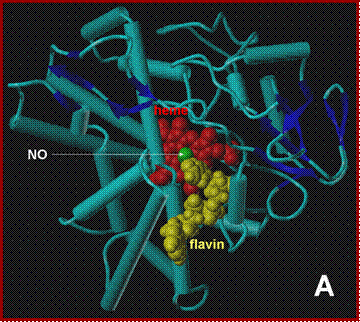
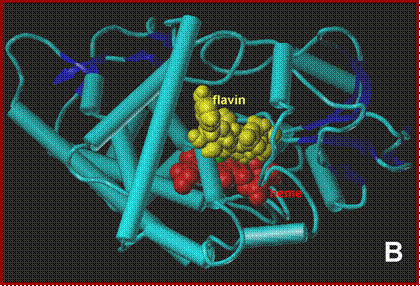
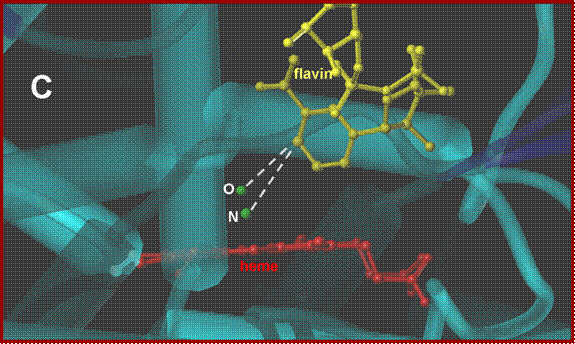
NADH docking onto the P450nor ferric-NO adduct. A:
View perpendicular to the solvent-exposed heme. B:
side-view, along the NADH-binding crevice. C: close-up of the active
side. Protein backbone-blue, heme-red,
NO-green, NADH-yellow.
•
A one-electron
mechanism, involving [FeNO]7 as
intermediate, is disfavored by the strong endothermicity
of the [FeNO]7 ® [FeNO]8 step,
and by the fact that [FeNOH]8 is less
stable than its isomer [FeN(H)O]8
•
A hydride
(two-electron) mechanism (initially proposed by Averill) is favored by the
above arguments and by experimentally-observed NADH/NADD kinetic isotope
effects
•
The docking results
suggest that the oxygen atom in the [FeNO]6 complex of P450nor is in a better position
than the nitrogen atom to receive the hydride from NADH.
•
The 444-nm intermediate
is proposed to be [FeNOH]8
(or, cf. subsequent results from other computational groups, the diprotonated version, [FeN(H)OH]8)
•
The NADH-binding
crevice identified by the docking results contains two residues previously
proven by site-directed mutagenesis to be involved in NADH binding