Nitric
oxide reduction by non-heme iron systems (FprA)
Nitric oxide is
ubiquitous in living organisms and serves roles ranging from signal
transduction to nitrate-based respiration. Nitric oxide can also be toxic. A
novel class of bacterial proteins, FprA (type A flavoproteins, and their congeners flavorubredoxins),
has recently been described, which catalyzes the detoxifying reduction of
nitric oxide (NO) to nitrous oxide (N2O):
2e-
+ NO +
2H+ --> N2O + H2O (nitric oxide reductase).
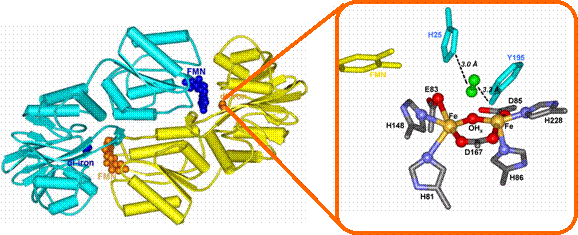
FprAs feature a histidine and carboxylate-ligated
diiron active site, similar to those in proteins
known to perform dioxygen activation via O-O bond
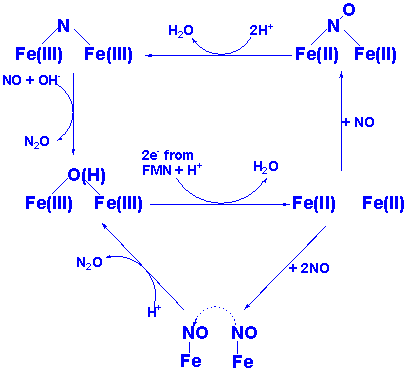
cleavage. We have been engaged in computational as well as experimental efforts
to identify the catalytic mechanism, proposing that enzymatic nitric oxide
reduction proceeds via a “super-reduced” Fe(II)-NO-
intermediate. Depicted below is the structure of FprA,
as well as two alternative mechanisms for NO reduction.