Radu Silaghi-Dumitrescu
BIOORGANOMETALLIC COMPLEXES RELEVANT TO THE “PUSH EFFECT” IN
HEMOPROTEINS.
Bioorganometallic
iron-aryl and iron-alkyl adducts of native hemoproteins
are known to contain an S = 1/2
ferric center s-bonded
to a carbon atom. Such aryl adducts of several hemoproteins
have allowed extensive probing of the topology of the respective heme active sites.
While spectroscopic data is available for most hemoprotein
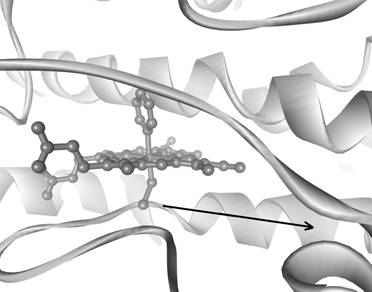
organometallic complexes, only the phenyl adduct of cytochrome P450 has been characterized by X-ray
crystallography (structure shown below).
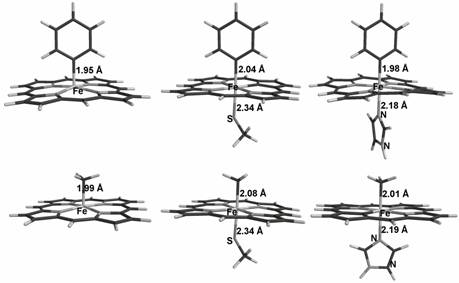
Geometry
optimization results for phenyl and methyl adducts of thiolate-ligated,
imidazole-ligated, and non-axially ligated porphyrin
models.
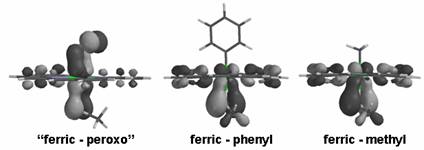
Molecular orbitals
illustrating p bonding
between iron and sulfur, in complexes examined here (ferric-methyl,
ferric-phenyl) as well as in a formally ferric-peroxo complex (“ferric-peroxo”,
with the axial ligand=SCH3).
Geometry optimization results using
non-hybrid density functional theory, for models of the known phenyl and methyl
adducts of heme-thiolate and heme-histidine
enzyme active sites, are in good agreement with
previously available experimental data. These bio-organometallic
complexes can be considered “s-only”
mimics of dioxygen, peroxo,
and superoxo complexes. A comparison of the Fe-C bond
lengths in these phenyl/methyl-heme complexes as a
function of the axial ligand allows a direct quantitative comparison of the trans (“push”) effect of the thiolate and histidine axial ligands in hemoproteins, without
any interference from p effects.
This allows us to estimate the p
contribution to the trans effect in the related,
previously examined, heme-dioxygen/peroxo complexes
with thiolate and histidine axial ligands. The phenyl
adduct also offers a previously unexplored explanation for the tilting of the
axial ligands in hemoproteins.
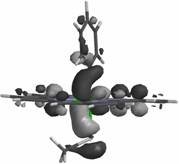
HOMO-2
orbital of the ferric-phenyl adduct, illustrating a s interaction between a carbon-based orbital and an
iron eg orbital.