PN-III-P1-1.1-PD-2016-0121
Ctr. 14 din 02/05/2018
Dr. Augustin C. Mot – project director
Prof. Dr. Costel Sarbu – mentor
Implementation period: May 2018 – April 2020
Advanced chemometric methods applied in plant metabolomics in water stress
Water stress is one of the major environmental factors that affects quality and crop production worldwide due to the lack of water or on the other hand, to the seasonal waterlogging or submergence stress (floods, hypoxic stress). Both of these water-related risks have enormous worldwide economic impact due to climate change, therefore, investigation of plants ability to resist in such stress is of great economic value. The profound understanding at the biochemical and molecular level in water stress are far from being explained and such understanding is crucial. Nonsymbiotic plant hemoglobins (nsHbs) are thought to be involved in many sorts of stress regulation, especially hypoxic response from the flooding stress via the NO dioxygenase activity, however, drought stress could also be envisaged to be regulated, at least in part by nsHbs. This proposal aims to track the metabolome in case of water stress and the involvement of nsHbs in plant adaptation and tolerance for these conditions using Arabidopsis thaliana as plant model and advanced chemometric methods. This direction, by the use of HPLC-MS, NMR analytical techniques and advanced multivariate chemometric methods as well as detection and characterization of metabolic by-products (ROS and RNS) using EPR techniques supports the biological relevance of the project. Comparison of the traditional chemometric methods in plant metabolomics with more performant methods of discrimination (fuzzified methods) in the light of their performances such as robustness, pattern recognition, outlier identification, in plant metabolomics is the analytical framework within these investigations are planned to be done.
Objectives:
Ř Characterization of the metabolome of A. thaliana in nsHbs modified lines (both mutants and overexpressing lines). This will imply a methodical use of HPLC-MS and NMR techniques and chemometric methods.
Ř Detection and characterization of metabolic by-products (such as ROS and RNS: hydrogen peroxide, superoxide and related species) of nsHbs (all three classes) modified plants in case of water stress, using EPR techniques and spin-traps and the involvement of the nsHbs.
Ř Comparison of the traditional chemometric methods in plant metabolomics with more performant methods of discrimination (fuzzy clustering, fuzzy principal component analysis, fuzzy-discriminant analysis), in the light of their performances such as robustness, pattern recognition, outlier identification in plant metabolomics.
Impact:
ü Explaining in more detail and making predictions on the main plant metabolic pathways that are involved in water stress.
ü Identifying the most important physiological roles involving nsHbs in water stress (including drought).
ü Setting the concept of fuzzy robust methods in plant metabolomics in a more solid framework.
ü Identifying novel biomarkers that may be used for monitoring and controlling water stress in plants.
ü In the longer term, it is foreseen that the results provide novel occasions for rational crop plans to improve plant water stress tolerance with high economic and agricultural impact.
Results and benefits:
1. Articles:
1. Augustin C. Mot*, Cristina Coman, Niculina Hadade, Grigore Damian, Radu Silaghi-Dumitrescu, Hendrik Heering, “Yellow” laccase from Sclerotinia sclerotiorum is a blue laccase that enhances its substrate affinity by forming a reversible tyrosyl-product adduct, PLOS One, 2019, accepted
2. Anca D. Farcas, Augustin C. Mot*, Cezara Zagrean-Tuza, Madalina Ticolea, Bogdan Sevastre, Muhittin Kulak, Radu Silaghi-Dumitrescu, Alina Parvu, Remarkable rutin-rich Hypericum capitatum extract exhibits anti-inflammatory effects on turpentine oil-induced inflammation in rats, BMC Complementary and Alternative Medicine, 2019, 19:289.
3. Anca D. Farcas, Augustin C. Moț*, Alina E. Pârvu, Vlad Al. Toma, Mirel A. Popa, Maria C. Mihai, Bogdan Sevastre, Ioana Roman, Laurian Vlase, Marcel Pârvu, In Vivo Pharmacological and Anti-inflammatory Evaluation of Xerophyte Plantago sempervirens Crantz, Oxidative Medicine and Cellular Longevity, 2019, 5049643:13
*corresponding author
2. Conferences (Oral presentations):
1. Augustin C. Mot, Chemometric techniques applied in fast profiling of plants in abiotic stress, 46th International Conference of the Slovak Society of Chemical Engineering, 20-23 May 2019, Tatranske Matliare, Slovakia
2. Augustin C. Mot, Cezara Zagrean-Tuza, Assessment of reaction intermediates in biomolecular systems using isotope exchange effect, 12th International Conference Processes in Isotopes and Molecules, 25-27 September 2019, Cluj-Napoca, Romania
3. Purchased equipments:

1. Concentrator în flux de azot/argon

2. Thermomixer

3. Vortex

4. Magnetic stirrers
Scientific report
regarding the implementation of the project PN-III-P1-1.1-PD-2016-0121 (contract no. 14 02.05./2018) with the title:
Advanced chemometric methods applied in plant metabolomics in water stress
within the period 2nd May 2018 – 30th April 2020
1. Water submergence treated Arabidopsis thaliana plants
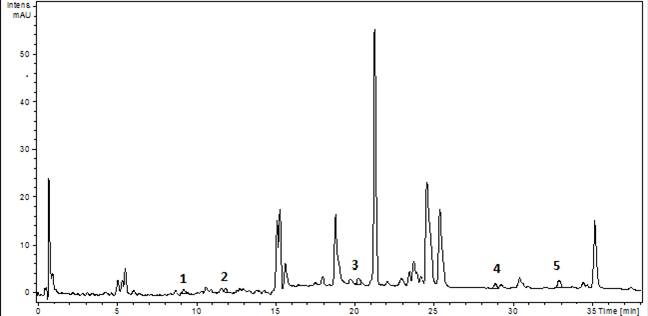
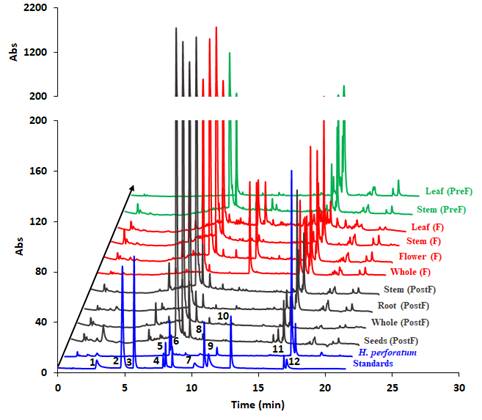
The experiments started by generating water submergence stressed plants of wild type (Arabidopsis thaliana, Columbia 0), GLB1 and GLB2 knock-out mutants, and overexpressing GLB1 and GLB2 homozygous genetic lines, 20 individuals for each line and their corresponding controls. The stress has been set on 6 weeks old plants that have been grown in Plant Chamber (21°C, 65 % relative humidity, 100 mol m-2 s-1, cycle of 16 h light, 8 h night). The collected plants (Figure 1) have been flash freeze in liquid nitrogen and freeze-dried under vacuum. The dried plant material (about 6-8% in weight of fresh collected plant leaves) were sequentially extracted with chloroform and the remaining residue was further extracted with methanol. The methanol extracts were analysed using HPLC-DAD chromatographic system, monitoring the absorbance at 242 and at 280 nm. The chromatogram of some representative samples is illustrated in Figure 2. Principal Component Analysis was applied on the chromatographic data monitored at 280 nm (Figure 3). Significant differences were observed in both water submergence stress treated samples and between wild type and mutants. Similar results were obtained using cluster analysis (Figure 4).

Figure 1. Some representative plants for each tested genetic lines after day 9th submergence and the control (untreated).
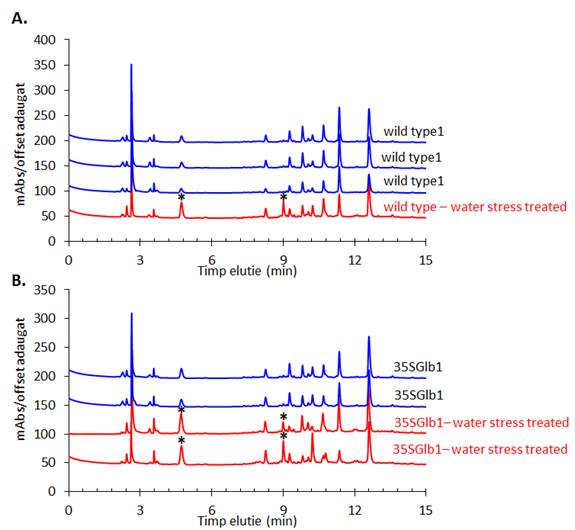
Figure 2. HPLC-DAD chromatogram of the methanolic extracts of the studied plants monitored at 280 nm. Metabolites that appear in higher amount in the treated samples than in the untreated ones are indicated using star (*).
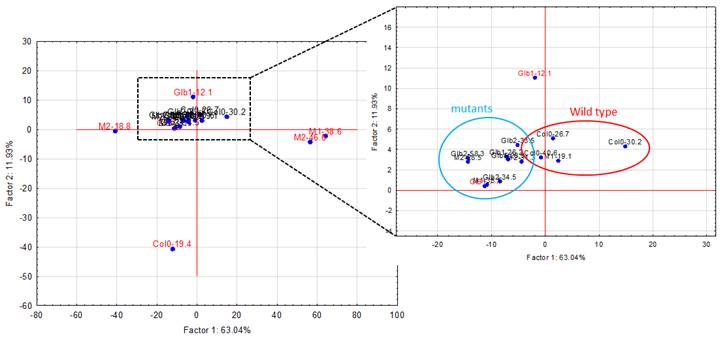
Figure 3. Principal component analysis (PCA) applied on the chromatograms from 242 nm.
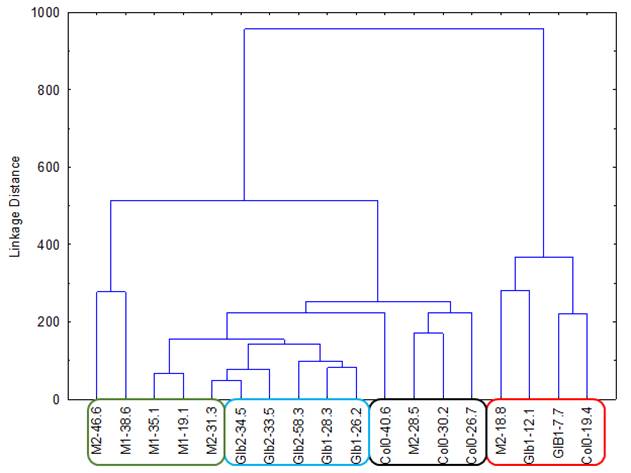
Figure 4. Cluster Analysis applied directly on 242 nm monitored chromatograms.
The chromatograms monitored at 242 nm reveal, besides the two metabolites at ca. 9 and 5 min as found by 280 nm chromatogram, a new one at ca. 7.5 minutes (Figure 5). A further aim will be to identify what is the nature of these compounds. The chromatographically analysed samples were also investigated for antioxidant activity evaluation using the ABTS radical bleaching assay (Figure 6). Water submergence treated samples have significantly lower antioxidant activity indicating that the water treatment induces oxidative stress that alter the redox balance status of the plants. In addition, some dried plant material was extracted using three other solvents: t-butyl-methyl-ether, methanol and isopropanol. The optimal solvent system used for extraction was determined by HPTLC chromatography (Figure 7). Chloroform appeared to be the best solvent for lipids extraction. The plate was eluted with petroleum ether/diethyl ether/formic acid, 7/3/0.6, v/v/v and detected with 10% CuSO4 (w/w) in 8% phosphoric acid (w/v) followed by incubation at 170 °C.
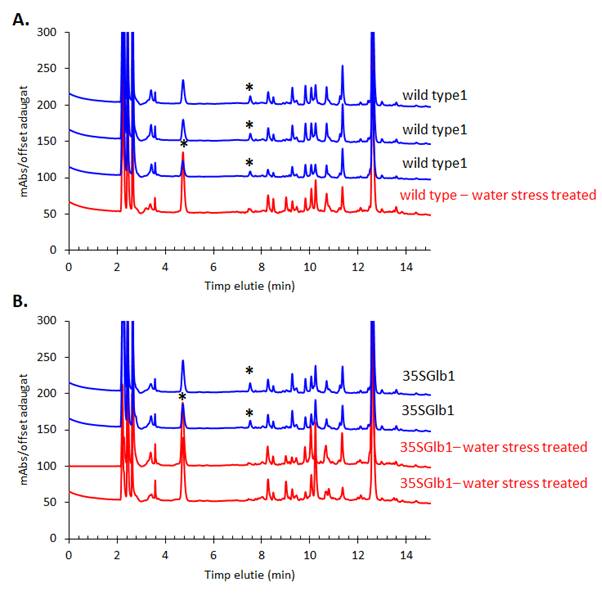
Figure 5. HPLC-DAD chromatogram of the methanolic extracts of the studied plants monitored at 242 nm. Metabolites that appear in higher amount in the treated samples than in the untreated ones are indicated using star (*).
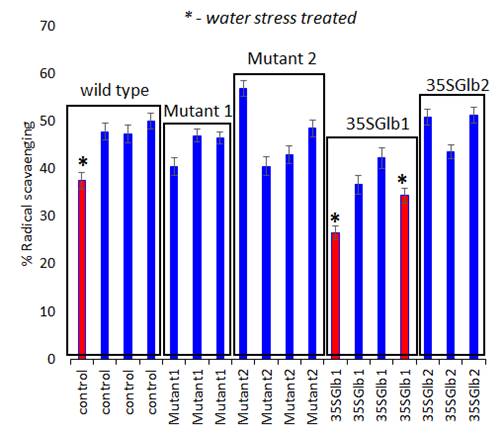
Figure 6. Antioxidant activity determination (using ABTS bleaching assay, 10 minutes’ reaction time) of the studied chromatographically analysed samples. Water submersed treated samples have significantly lower activity (red coloured).
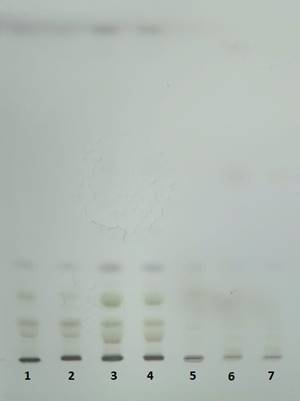
Figure 7. HPTLC plate (10 x 10 cm, Silicagel 60) after separation of the lipids extracted samples (wild type) using t-butyl-methyl ether (1,2), chloroform (3,4) and isopropanol (5,6,7).
2. NMR analysis of the water submerged Arabidopsis thaliana plants
The plants obtained after water submergence stress were freeze-dried and extracted with deuterated methanol (d6) and D2O (10%). The extracts were analysed using a 600 MHz 1H NMR spectrometer and the results are presented in Figures 8-13.
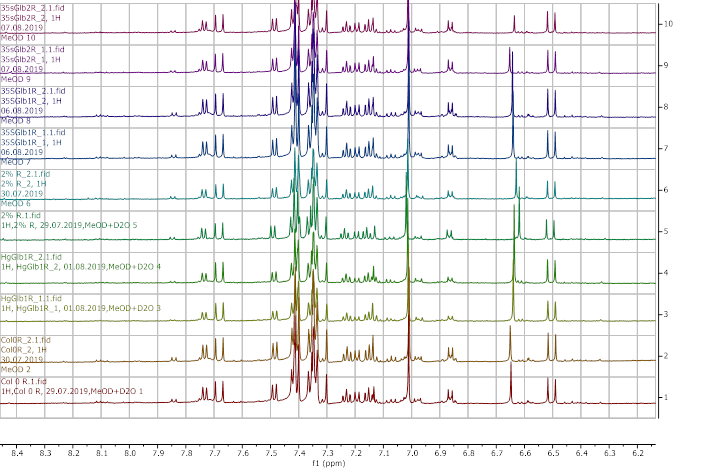
Figure 8. 1H NMR (600 MHz) of the control unsubmerged A. thaliana extracts in deuterated methanol (6.2-8.4 ppm region). Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
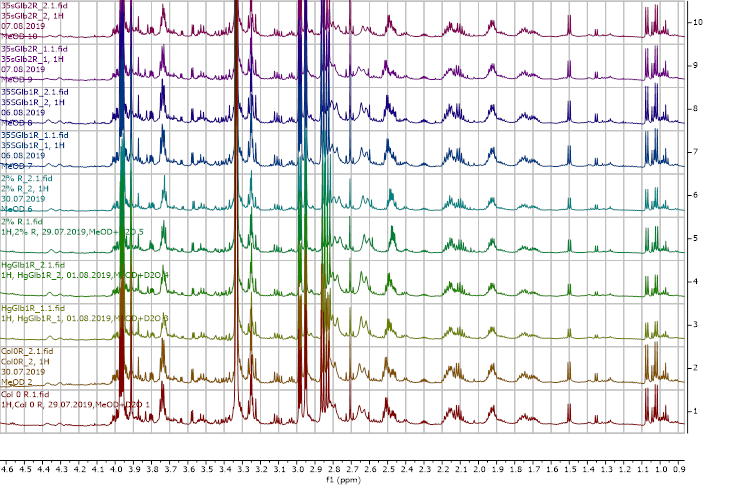
Figure 9. 1H NMR (600 MHz) of the control unsubmerged A. thaliana extracts in deuterated methanol (0.9-4.6 ppm region). Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
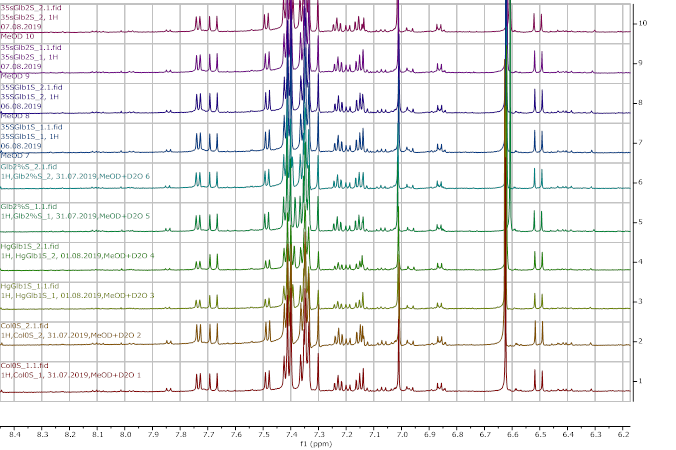
Figure 10. 1H NMR (600 MHz) of the submerged A. thaliana extracts in deuterated methanol (6.2-8.4 ppm region). Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
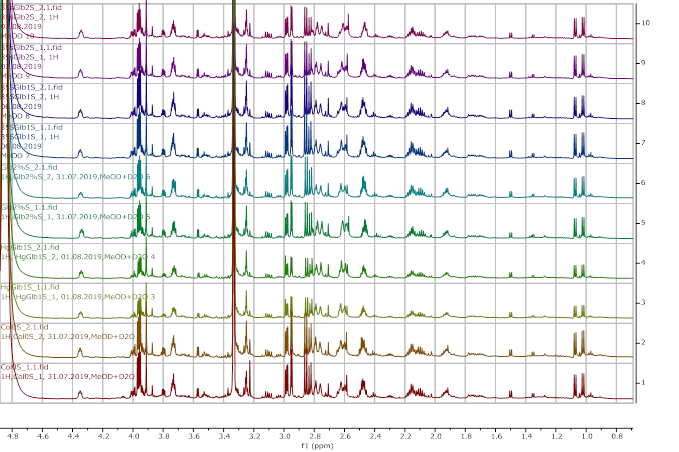
Figure 11. 1H NMR (600 MHz) of the submerged A. thaliana extracts in deuterated methanol (0.9-4.6 ppm region). Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
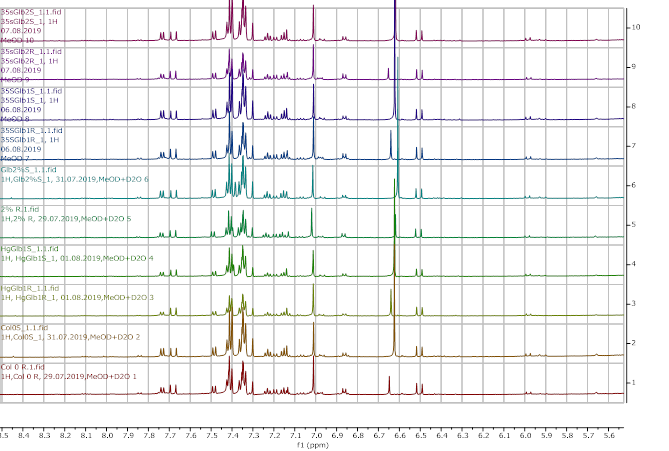
Figure 12. 1H NMR (600 MHz) of the submerged vs. control (unsubmerged) A. thaliana extracts in deuterated methanol (5.6-8.4 ppm region). S – submerged, R- control. Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
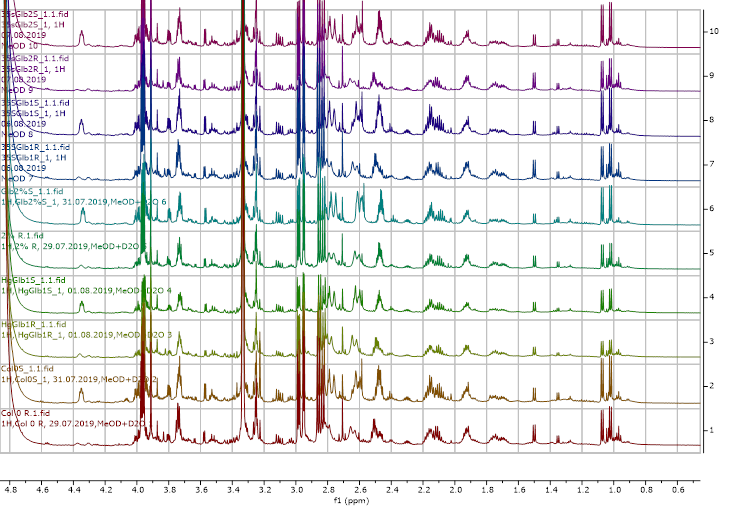
Figure 13. 1H NMR (600 MHz) of the submerged vs. control (unsubmerged) A. thaliana extracts in deuterated methanol (5.6-8.4 ppm region). S – submerged, R- control. Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
As it can be easily noticed, the controls and the submerged extracts are well reproducible. It is very interesting that distinct modified peaks were identified in case of the submerged plants, suggesting important chemical compositions modification in case of water submergence (eg. 2.6 ppm, 4.4 ppm, etc.). In addition, this modification is well reproducible, again. Chemometric techniques will be applied on the digitized data in order to evaluate the significance of the two groups.
The water-deficit stress was set for Arabidopsis thaliana plants, and the plants are presented in Figure 14. As it can be noticed, a clear difference is observed between the studied genetic lines, indicating the importance role of the non-symbiotic plant haemoglobins that are played in this stress. The plant extracts will be analysed in order to identify the chemical constituents’ difference between the studied genetic lines.

Figure 14. The water-deficit stress plants (after 9 days of stopping the watering of the plants) of the studied. Col0- wild type, 35SGlb1,2-overexprising nsHbs, HgGlb1 and 2-/- are the two mutants for the nsHbs1 and 2, respectively.
3. Phytochemical analysis by HPLC-MS and phytoconstituents profiling using EPR spectroscopy of a xerophyte plant species1
For more practical reasons, a xerophyte plant species—a plant that is adapted to leave with very little water, in a desert-like region, Plantago sempervirens—was tested for phytochemical analysis, in contrast with other Plantago species from the same genus. It turned out that this species is by far more anti-inflammatory and antioxidant compare with its non-xerophyte counterparts. The results have been recently published in a complex study coordinated by the director of this project1.
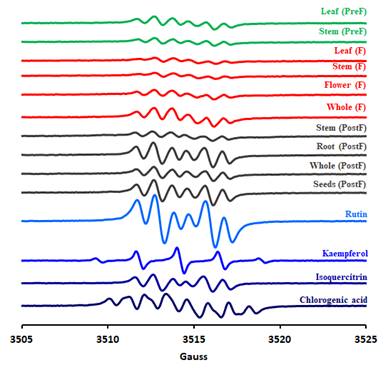
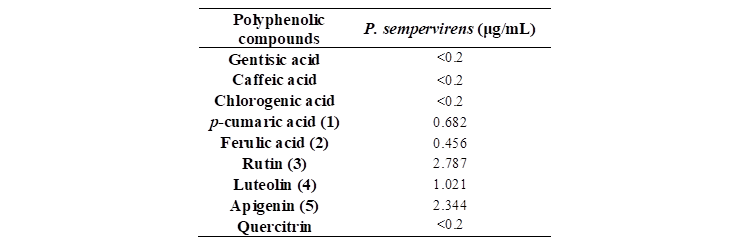
As shown in Table 1, Figures 15 and 16, chemical profile of P. sempervirens reveals the main compounds identified in each extract. By far, rutin, luteolin and apigenin were the main compounds in Plantago samples. Therefore, we found 1,021 μg/mL of luteolin; 6.48 ug/mL of apigenin and 2,34 μg/mL of rutin. P. sempervirens, by far was a rich extract because from the nine polyphenols quantified five were above the LOQ [apigenin (2,34 μg/mL), rutin (2,78 μg/mL), luteolin (1,02 μg/mL), acid ferulic (0,45 μg/mL) and acid p-coumaric acid (0,68 μg/mL)].1.
Table 1. The HPLC polyphenolic compounds content determination in the studied species (ug/mL extract)1
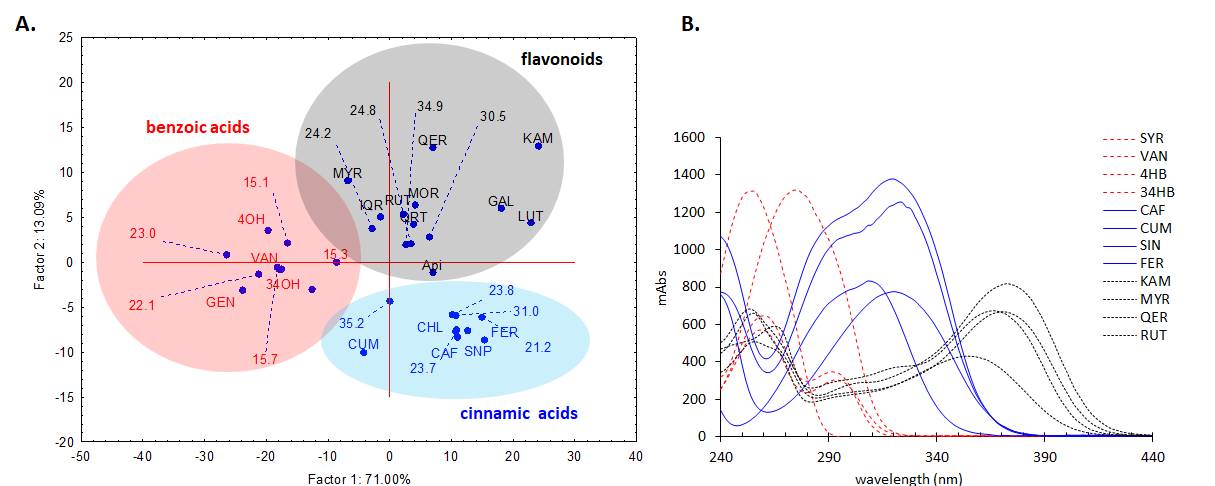
In order to further analyse the major components from the chromatogram that were not identified in MS or based on retention time of the standards, UV-vis molecular spectra of these major chromatographic peaks (cca. 90% of total chromatographic area AU×min, for 280 nm chromatogram) were exported and analysed together with the spectra of twenty known standards using a chemometric approach. PCA analysis of the exported spectra reveals three distinct groups based on the three classes of polyphenols, i.e. benzoic acids, cinnamic acids and flavonoids, which are in general, the main phytoconstituents responsible for antioxidant activity in plant extracts (Figure 15A). Most of the tested compounds (cca. 80% of the chromatographic area, the four most intense peaks) are belonging to cinnamic acids group which present a distinct UV-vis spectrum profile. The assignments of each peak spectrum was also checked using the build-in soft (Agilent Chemstation) of the HPLC chromatograph using house-built-in data base of tens of polyphenolic standards. For this purpose, a score of spectral similarity higher than 90% for any compound (with a given standard) from those three classes was an indication that the tested compound does belong to a certain class. Spectral profiles of some representatives of these classes are shown in Figure 15B1. The HPLC results for the P. sempervirens extract is presented in Figure 16.